Phospho-Pin1 (Ser16) Antibody - #AF3430
製品説明
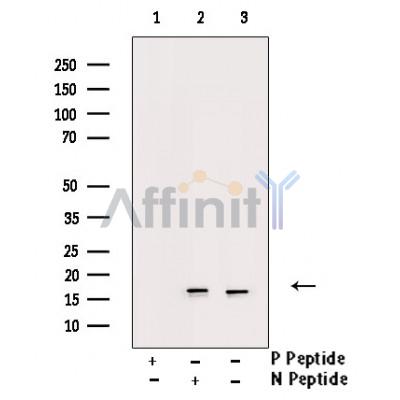
*The optimal dilutions should be determined by the end user. For optimal experimental results, antibody reuse is not recommended.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
引用形式: Affinity Biosciences Cat# AF3430, RRID:AB_2834872.
折りたたみ/展開
DOD; DODO, Drosophila, homolog of; FLJ40239; FLJ77628; MGC10717; NIMA interacting 1; Peptidyl prolyl cis trans isomerase NIMA interacting 1; Peptidyl prolyl cis/trans isomerase NIMA interacting; Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1; Peptidyl-prolyl cis-trans isomerase pin1; Peptidylprolyl cis/trans isomerase NIMA interacting 1; Pin 1; Pin1; PIN1_HUMAN; PPIase Pin1; Prolyl isomerase; Protein (peptidylprolyl cis/trans isomerase) NIMA interacting 1; Protein NIMA interacting 1; Rotamase Pin1; UBL 5; UBL5;
免疫原
A synthesized peptide derived from human Pin1 around the phosphorylation site of Ser16.
The phosphorylated form at Ser-71 is expressed in normal breast tissue cells but not in breast cancer cells.
- Q13526 PIN1_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MADEEKLPPGWEKRMSRSSGRVYYFNHITNASQWERPSGNSSSGGKNGQGEPARVRCSHLLVKHSQSRRPSSWRQEKITRTKEEALELINGYIQKIKSGEEDFESLASQFSDCSSAKARGDLGAFSRGQMQKPFEDASFALRTGEMSGPVFTDSGIHIILRTE
種類予測
Score>80(red) has high confidence and is suggested to be used for WB detection. *The prediction model is mainly based on the alignment of immunogen sequences, the results are for reference only, not as the basis of quality assurance.
High(score>80) Medium(80>score>50) Low(score<50) No confidence
研究背景
Peptidyl-prolyl cis/trans isomerase (PPIase) that binds to and isomerizes specific phosphorylated Ser/Thr-Pro (pSer/Thr-Pro) motifs. By inducing conformational changes in a subset of phosphorylated proteins, acts as a molecular switch in multiple cellular processes Ref. 21). Displays a preference for acidic residues located N-terminally to the proline bond to be isomerized. Regulates mitosis presumably by interacting with NIMA and attenuating its mitosis-promoting activity. Down-regulates kinase activity of BTK. Can transactivate multiple oncogenes and induce centrosome amplification, chromosome instability and cell transformation. Required for the efficient dephosphorylation and recycling of RAF1 after mitogen activation. Binds and targets PML and BCL6 for degradation in a phosphorylation-dependent manner. Acts as a regulator of JNK cascade by binding to phosphorylated FBXW7, disrupting FBXW7 dimerization and promoting FBXW7 autoubiquitination and degradation: degradation of FBXW7 leads to subsequent stabilization of JUN. May facilitate the ubiquitination and proteasomal degradation of RBBP8/CtIP through CUL3/KLHL15 E3 ubiquitin-protein ligase complex, hence favors DNA double-strand repair through error-prone non-homologous end joining (NHEJ) over error-free, RBBP8-mediated homologous recombination (HR).
Phosphorylation at Ser-71 by DAPK1 results in inhibition of its catalytic activity, nuclear localization, and its ability to induce centrosome amplification, chromosome instability and cell transformation.
Nucleus. Nucleus speckle. Cytoplasm.
Note: Colocalizes with NEK6 in the nucleus (PubMed:16476580). Mainly localized in the nucleus but phosphorylation at Ser-71 by DAPK1 results in inhibition of its nuclear localization (PubMed:21497122).
The phosphorylated form at Ser-71 is expressed in normal breast tissue cells but not in breast cancer cells.
The WW domain is required for the interaction with STIL and KIF20B.
研究領域
· Organismal Systems > Immune system > RIG-I-like receptor signaling pathway. (View pathway)
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.